In the realm of analyzing test substances, the preliminary treatment of samples presents a complex challenge. This aspect is pivotal within the scope of analysis and detection efforts, constituting a significant facet for identifying the origins of detection inaccuracies. Consequently, this article aims to synthesize four fundamental sample pretreatment techniques employed in detection endeavors of Atomic Absorption Spectrophotometer (AAS) . Additionally, it delves into the foundational detection techniques for six categories of routine samples. These methodologies offer practicality and serve as valuable references for users of Atomic Absorption Spectrophotometers (AAS).

The Four Core Techniques for General Sample Pretreatment in AAS
Wet Digestion Method
For samples approximately ranging from 0.1000 to 0.5000g, prevalent mixed acids are utilized, with varying ratios as follows:
HNO3 : HClO3 = 5 : 1
HNO3 : H2SO4 = 5 : 1
HNO3 : HCl = 5 : 1
Pure HNO3
Note: Volatile (such as acetone, ether, ethanol, etc.) and flammable, explosive substances must be absent during the digestion process. While the wet digestion method is widely employed, its operational specifics will not be reiterated here.
Dry Ashing Method
Suitable for sample sizes typically ranging from 2.000 to 5.000g, this method involves careful treatment to prevent volatilization. The process entails placing the sample in a porcelain crucible, moistening it with a few drops of water, adding a small quantity of concentrated nitric acid, heating it gently to carbonize, and subsequently ashing it in a muffle furnace at around 550°C for 2 to 4 hours. Post-cooling, the residue is dissolved with additional acids plus HNO3 (1:1), which varies depending on the sample. The solution is then filtered, adjusted to volume, and readied in 10mL, 25mL, and 50mL portions for subsequent use.
High-Pressure Vessel Method (Utilizing Polytetrafluoroethylene Lid-Made Tanks)
When dealing with samples below 0.3000g, this method involves introducing 6mL of mixed acid and 1mL of HF (H2O2) to the sample. The lidded autoclave is then sealed, and heating is performed at 160°C for 5 hours. After cooling, the sample is filtered, adjusted to volume, and set aside for future use.
Microwave Digestion Method
In this approach, commonly employed mixed acids encompass:
HNO3 : HClO3
HNO3 : H2SO4
Pure HNO3
It is essential to select the appropriate acid for specific digestion depending on the sample. Readers are encouraged to make suitable choices as per their requirements.
Conventional AAS Analysis and Detection Approaches for Diverse Samples
Analysis of Pb, Cd, As, Mo, Cr, etc. (Graphite Furnace AAS Method)
Pb: Extract 1.0mL of the sample, dilute it to 10mL with 1% HNO3. Linear range: 0~20ng/mL, drying temperature: 80~100°C, ashing temperature: 200°C, atomization temperature: 1500°C.
Cd: Extract 1.0mL of the sample, dilute it to 10mL with deionized water. Linear range: 0.1~0.4ng/mL, drying temperature: 80~100°C, ashing temperature: 200°C, atomization temperature: 1800°C.
As: Extract 1.0mL of the sample, add 100μL of Ni (2mg/mL), dilute to 10mL with 1% HNO3. Linear range: 0~4ng/mL, drying temperature: 80~100°C, ashing temperature: 200°C, atomization temperature: 2200°C.
Mo: Prepare a 1.0mL sample diluted with 1% HNO3 to 10mL. Using Pd as a modifier, the linear range is 0-20ng/mL. Drying temperature: 80-100°C, ashing temperature: 1200°C, atomization temperature: 2600°C.
Cr: Sample 1mL, dilute to 100mL with deionized water, and proceed with appropriate testing. Linear range: 0~40ng/mL, drying temperature: 80~100°C, ashing temperature: 1000°C, atomization temperature: 2600°C.
Analysis and Testing of Se, K, Na, Ca, Mg, etc. in Plant Samples
Plant samples are subjected to washing, drying, fixation at 150°C for 10 minutes, subsequent drying at 70°C, and final comminution before analysis.
Se in forage grass: Extract 1mL of the sample, add 100μL of Ni(NO3)2 improver (3mg/mL), dilute to 10mL with 1% HNO3 and 0.1% Triton (1:1). Linear range: 0~20ng/mL, drying temperature: 80~100°C, ashing temperature: 1000°C, atomization temperature: 2400°C.
K and Na in plants: Weigh 0.2000g of the sample, add 10mL of mixed acid, extract in a constant-temperature water bath at 90°C, filter to 50mL volume, and proceed with suitable sampling and testing.
Ca and Mg in plants: Incinerate the sample at 550°C, dissolve with hydrochloric acid (1+1), adjust to 50mL volume (with 5% CsCl2 modifier 2 mL), and proceed with appropriate sampling and testing.
Beryllium Analysis in Mushrooms and Tea (Transverse Heating AAS Method)
Prepare the sample by washing, drying, fixation at 105°C, subsequent drying at 70°C, cooling, and crushing through a 60 mesh sieve.
Weigh 1.0000g of sample, add 15mL of HNO3, 1mL of H2SO4, digest at a low temperature, dilute to 25mL, and proceed with suitable sa
mpling for testing. Linear range for Be: 0~8ng/mL. Test conditions: drying temperature: 130°C, ashing temperature: 1500°C, atomization temperature: 2300°C.
Germanium Testing in Beverages (Utilizing Transversely Heated Flat Graphite Tubes)
Test outcomes indicate a linear range of 0~200ng/mL, using Ni(NO3)2 improver (50μg/mL) in a 1% HNO3 medium. Instrument conditions: drying temperature: 130°C, ashing temperature: 800°C, atomization temperature: 2000°C.
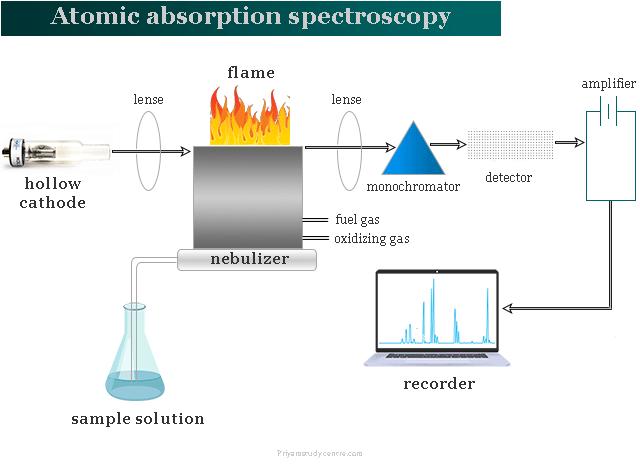
Calcium Detection in Steel Slag (Typically Employing Flame AAS Method)
For samples above 200 mesh, weigh 0.0500g into a 50mL PTFE crucible. Dissolve it with a water:nitric acid:hydrofluoric acid ratio of 5:8:6. Add sulfuric acid, heat, and adjust volume for analysis. To mitigate niobium interference, include
Triton-100 (20%), Vc (0.1 M/L), and 2% HNO3 as improvers.
Determination of Metal Elements in Blood Samples
Human blood encompasses diverse trace constituents, including heavy metal elements. Various detection methods can discern these components, with AAS being particularly effective. Specific digestion methods are elaborated in sections "2, 3), (3)." For instance:
Cu determination in serum (flame AAS method): Dilute 0.8mL of serum with 1% HNO3 to 10mL for testing.
As determination in serum (graphite furnace AAS method): Dilute with 1% HNO3 and utilize Ni(NO3)2 as a matrix modifier.
Cd determination in serum (graphite furnace AAS method): Process 2mL of serum with HNO3 and H2O2 for analysis.
Fe and Cu determination in albumin (flame AAS method): Utilize 1mL of the sample with mixed acid, adjust volume, and proceed with appropriate sampling.
Germanium determination in whole blood (graphite furnace AAS method): Dilute 1.0mL of venous blood, utilize a matrix modifier mixture, and perform analysis at defined temperatures.
This comprehensive overview highlights essential techniques for accurate analysis and detection of various samples by using atomic absorption spectrometry.