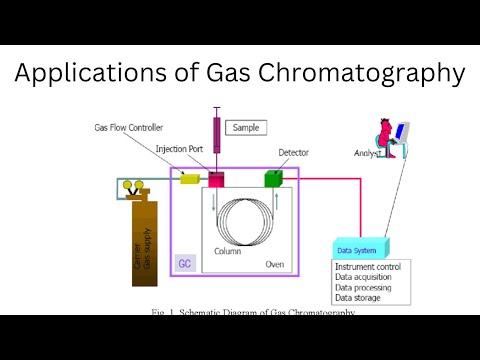
In order for a piece of equipment to successfully complete an elemental analysis, it will first require that a sample be prepared in advance. In order for the instrument to be able to perform the analysis, the specimen must first be converted from its original liquid or solid state into a form that it can evaluate. In the case of flame AAS, this is accomplished through the atomization of the sample, which, in the end, results in the creation of a fine mist dispersion. After that, this mist is made to pass through a flame, which further breaks apart any molecular bonds that may have been lingering after the previous step. The term "atomization" is used to refer to this particular process throughout the industry. This is done in order to ensure that the analysis carried out by the AAS is accurate.
After that, the sample is subjected to a procedure in which it is made to go through a process in which it is exposed to a source of radiation, which, in the overwhelming majority of instances, is a source of light. The procedure will be repeated as many times as necessary until the sample has been analyzed. The spectrometer serves as the light source. The light intensity across a light spectrum can be reduced in one or more of its regions as a direct result of absorption occurring in any of those regions. This is something that, no matter where on the spectrum you look at it from, is conceivable in some form or another. This decreased intensity is characteristic of a particular element, and it helps in identifying that element as well as determining the concentration of that element. Case in point:In order for the AAS device to perform its functions in the manner for which they were designed, it is necessary to make use of specialized instruments such as atomizers and monochromators.
After that, the analyte is excited by a number of different sources of light, which ultimately results in the emission of a spectrum that is comprised of waves of varying lengths. Spectroscopy is a method that is used to analyze chemical substances. Following the dispersion of these wavelengths, the detector in the AAS instrument is the component that is responsible for measuring the intensity of the wavelength. It is possible to determine the concentration of the element of interest because the concentration of an element can be determined, and because the concentration of an element is a function of the intensity of its wavelength, it is possible to determine the concentration of the element of interest. Because of this, one is able to calculate the proportion of the element of interest that is present in the sample. This makes it possible to conduct quantitative analyses on samples for which the characteristics are unknown.
The term "flame atomic absorption spectrometry" (which is also the full name of the technique) is referred to by its abbreviation "FAAS."Sodium, potassium, calcium, and magnesium are a few examples of these types of elements. Iron is another. It has gained widespread acceptance in a wide variety of industries, all of which continue to take advantage of the one-of-a-kind and industry-specific benefits that are made available by the technology that underpins this innovation. This innovation has been a game-changer for many businesses.
The first thing that happens during the analysis process is that liquid samples are drawn into a vacuum. After that, the samples are introduced into a flame using a spray chamber, which is the second step in the process. The flame is generated by typically utilizing air and acetylene gases or nitrous oxide and acetylene gases, and as a direct result of this, the sample is broken down, vaporized, and atomized. Note: Once that step has been finished, the light will be shone through the flame so that measurements can be taken while the atomization process is taking place. In order to ensure that the light path is consistently and flawlessly aligned for the purposes of this analysis, high-performance optics and accurate monochromator operation are utilized. This is done so that an examination can be carried out as the intended purpose.
Graphite furnace atomic absorption spectrometry is what the abbreviation GFAAS stands for when it's written out.
Graphite furnace atomic absorption spectrometry, also known as GFAAS, is a well-established analytical technology that can measure a large number of elements at concentrations as low as parts per billion. The acronym GFAAS stands for graphite furnace atomic absorption spectrometry. atomic absorption spectrometer is only necessary to use a very minute portion of the sample, and the vast majority of the time, only a few microliters of sample are directly injected into a graphite cuvette. After the matrix has been removed from the sample in order to get it ready for the atomization process, the sample is then dried with the assistance of controlled electrical heating applied to the cuvette. This completes the atomization preparation process. Hollow cathode lamps are tasked with the responsibility of producing a particular elemental light output.
Work is done in preparation in order to get the samples ready for both the FAAS and the GFAAS.
menu
menu
Menu