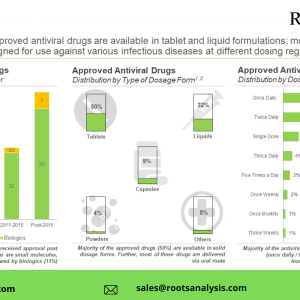
Given their low immunogenicity, diversified targets and ability to offer higher biosafety, the adeno-associated viral vectors are actively being evaluated for the development of various therapies for the treatment of chronic diseases and infections
Roots Analysis has announced the addition of “Adeno Associated Viral Vector Market, 2022-2035” report to its list of offerings. The report features an extensive study on the current landscape and the likely future potential of adeno-associated viral vector market over the next decade.
To get a sample copy of the latest market report, visit
https://www.rootsanalysis.com/reports/adeno-associated-viral-vector-market/request-sample.html
The study also features an in-depth analysis, highlighting the capabilities of various stakeholders engaged in this domain. Amongst other elements, the report features:
- A general overview of the various types of viral and non-viral vectors. It includes a detailed discussion on structure and design, life cycle and applications of adeno-associated viral vectors. The chapter concludes with a discussion on the various advantages and challenges related to adeno-associated viral vectors.
- A detailed overview of the overall market landscape of adeno-associated viral vector-based therapies, including information on their phase of development, key therapeutic areas, type of gene / molecule targeted, type of therapy, type of gene delivery method used, route of administration and special drug designation(s) awarded.
- An overview of the current status of the market with respect to the players engaged in the manufacturing of adeno-associated viral vectors, featuring information on the year of establishment, company size, location of headquarters, type of product manufactured, location of manufacturing facilities, type of manufacturer, scale of operation and application area.
- An overview of the technologies offered / developed by the companies enagaged in this domain, including a detailed analysis based on the type of technology, scale of operation, application and most prominent players within this domain, in terms of number of technologies.
- Detailed profiles of marketed and late stage (phase II / III and above) adeno-associated viral vector based therapies, along with information on the development timeline of the therapy, current development status, mechanism of action, affiliated technology, patent portfolio, dosage and manufacturing details, as well as details related to the developer company.
- Tabulated profiles of players having capability to manufacture adeno-associated viral vectors (shortlisted based on proprietary criterion). Each profile features an overview of the company / organization, its financial performance (if available), vector manufacturing related capabilities and an informed future outlook.
- A region-wise, company competitiveness analysis, highlighting key players engaged in the manufacturing of adeno-associated viral vector, based across key geographical areas, featuring a four-dimensional bubble representation, which takes into consideration supplier strength, manufacturing strength, service strength and company size.
- A detailed competitiveness analysis of adeno-associated viral vector platforms, taking into consideration the supplier strength and key technology specifications, such as purpose of technology, scale of operation and application area(s).
- An in-depth analysis of completed, ongoing and planned clinical studies, based on several relevant parameters, such as trial registration year, trial status, trial phase, target therapeutic area, geography, type of sponsor, prominent treatment sites and enrolled patient population.
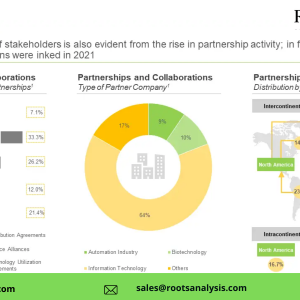
- An analysis of recent collaborations and partnership agreements inked in this domain since 2017; it includes details of deals that were / are focused on the manufacturing of vectors, which were analyzed on the basis of year of partnership, type of partnership, therapeutic area, type of partner and regional distribution of partnerships.
- An insightful analysis of the companies that have likelihood of establishing partnerships with adeno-associated viral vector and gene therapy product manufacturers, based on several parameters, such as developer strength, product strength, therapeutic area and pipeline strength.
- An in-depth analysis of various patents that have been filed / granted for adeno-associated viral vector-based therapies since 2017, based on several relevant parameters, such as type of patent, publication year, regional applicability, CPC symbols, emerging focus areas, leading industry players (in terms of the number of patents filed / granted), and patent valuation.
- An analysis of the various start-ups engaged in the development of adeno-associated viral vectors-based therapies, based on relevant parameters, such as number of candidates in discovery, preclinical and clinical phases of development, number of patents and number of partnerships established.
- An insightful analysis, highlighting the various factors that need to be taken into consideration by adeno-associated viral vector manufacturers to facilitate decision making to manufacture their respective products in-house or engage the services of a CMO. Further, the analysis highlights all the key parameters that must be considered by players based on company sizes, while taking the aforementioned decision.
- Type of Therapy
- Gene Augmentation
- Immunotherapy
- Other Therapies
- Type of Gene Delivery Method Used
- Ex vivo
- In vivo
- Target Therapeutic Area
- Genetic Disorders
- Hematological Disorders
- Infectious Diseases
- Metabolic Disorders
- Ophthalmic Disorders
- Muscle Disorders
- Neurological Disorders
- Others
- Scale of Operation
- Preclinical
- Clinical
- Commercial
- Application Area
- Gene Therapy
- Cell Therapy
- Vaccines
- Geographical Regions
- North America
- Europe
- Asia-Pacific
- MENA
- Latin America
- Rest of the World
Key companies covered in the report
- Abeona Therapeutics
- Aldevron
- Oxford BioMedica
- Sanofi
- WuXi AppTec
- Yposkesi
For additional details, please visit https://www.rootsanalysis.com/reports/adeno-associated-viral-vector-market.html
You may also be interested in the following titles:
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415